Why is the lowest temperature in the universe limited to -273.15℃? Some people may not be able to answer, and it doesn’t matter if you can’t answer, let’s talk about this knowledge.

Temperature is a physical quantity used to express the degree of hot and cold of an object. If you want to discuss temperature, you must first define a unit of measurement for temperature. How to define it?
We know that at 1 standard atmosphere, both the freezing and boiling points of water are fixed, which obviously serves as a very good reference.
So in 1742, the Swedish physicist Anders Celsius proposed that at 1 standard atmosphere, the temperature of boiling water can be recorded as 0 degrees, and the temperature of ice water can be recorded as 100 degrees, The two can be divided into 100 equal parts, each equal part is 1 degree, and this is the origin of our commonly used temperature measurement unit – Celsius (°C).

(Anders Celsius)
When you see this, you must ask, is this the opposite? Isn’t the temperature of ice water 0 degrees and the temperature of boiling water 100 degrees? In fact, it was not the other way around. Celsius proposed this definition at the time to avoid negative numbers when measuring temperature because it is lower than the freezing point of water.
Later, people felt that this definition was very inconvenient. After all, intuitively, the larger the value, the higher the temperature. On the other hand, higher temperatures than boiling water also exist, and negative numbers are still inevitable, so people Simply reverse this definition, and it has been used to this day.
As early as the 16th century, the famous physicist Galileo discovered the phenomenon of thermal expansion and contraction of gases. With the definition of “Celsius” and the technology of accurately measuring temperature, people can study the effect of temperature on the volume of gas more deeply. .
In 1787, the French physicist Jacques Charles conducted experiments on a variety of gases. His experimental results showed that under the condition of constant pressure, the temperature of these gases increased by 1 °C, the volume The increase in is always a fixed value, approximately 1/273 of its volume at 0°C.
(Jacques Charles)
That is to say, if the volume of a gas at 0°C is 1 cubic meter, then when the temperature rises to 1°C, its volume will increase by about 0.00366 cubic meters (273 of its volume at 0°C). 1/1), that is, 1.00366 cubic meters. When it is raised to 2 °C, its volume will increase by 0.00366 cubic meters, which is 1.00732 cubic meters. Then it will be accumulated in the same way. For example, when the temperature is increased to 100 °C, this The volume of the gas mass increased to 1.366 cubic meters.
In 1802, British physicist Gay-Lussac proposed the “Charles’ Law” (also known as “Gay-Lussac’s Law”), which states that under constant pressure, the Volume is proportional to its temperature, and “1/273”, measured by Jacques Charles, is taken as the volumetric expansion coefficient of a gas at constant pressure.

(Guy-Lussac)
In the middle of the 19th century, human measurement technology has made great progress, and scientists have improved the accuracy of this expansion coefficient to 1/273.15 in the laboratory.
Imagine that, at constant pressure, for every 1°C increase in the temperature of a mass of gas, its volume increases by 1/273.15 of its volume at 0°C, then for every 1°C drop in the temperature of the gas, The reduction in volume is also 1/273.15 of its volume at 0°C.
After a simple calculation, it can be concluded that for a group of gas with an initial temperature of 0°C, when the temperature drops to -273.15°C, the volume of the gas is zero (provided that the pressure is constant). Obviously, a mass of gas with zero volume cannot exist, which means that -273.15°C is impossible to reach.
It can be seen that the special significance of the temperature value of -273.15°C is that it is the theoretical lower limit of the temperature of the universe calculated by scientists through theory and experiments.

(Lord Kelvin)
In 1848, Lord Kelvin, known as the “father of thermodynamics”, in his paper “On an Absolute Temperature Scale”, proposed a purely theoretical temperature scale that has nothing to do with the properties of thermometric substances. Set the theoretical lower limit of temperature, which is -273.15°C, as “absolute zero” and increment it in degrees Celsius.
Yes, this temperature scale is the later thermodynamic temperature scale, and its unit is K (Kelvin). = ℃ + 273.15” to convert, for example, 1℃ is equivalent to 274.15K.
In the following time, with the progress of science, people also became clear about the nature of temperature in the universe, which is actually the intensity of thermal motion of microscopic particles inside an object, so the temperature corresponding to “absolute zero” is of course “microscopic particles inside an object” The intensity of thermal exercise is zero.”
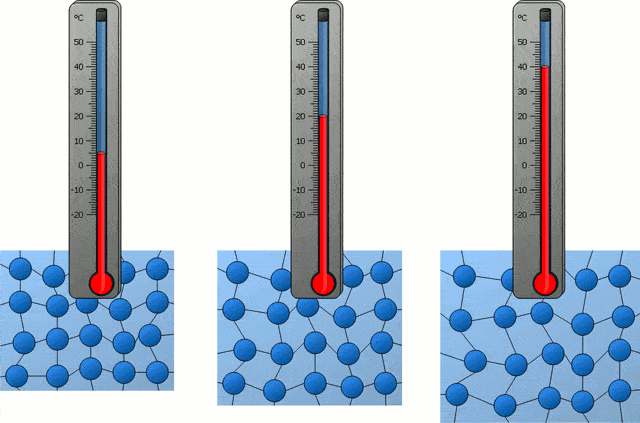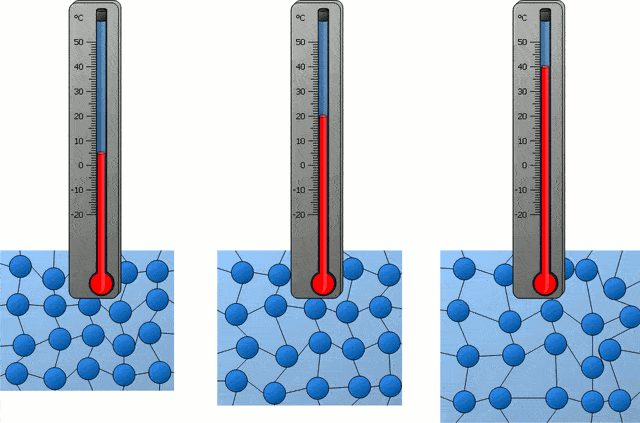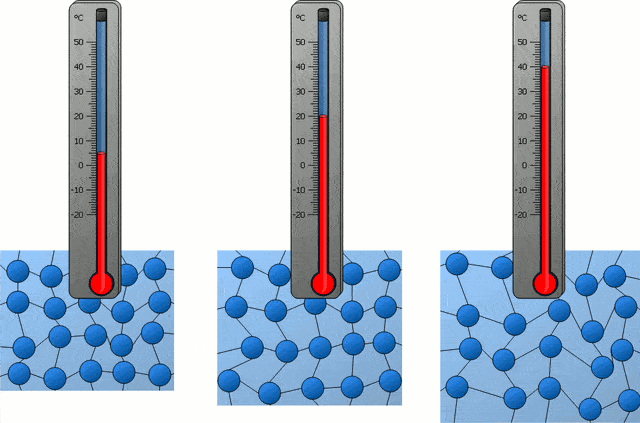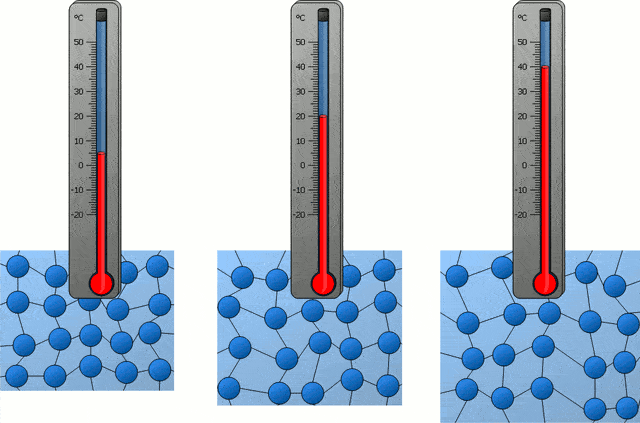
If all the microscopic particles inside an object are completely still, then the temperature of this object is “absolute zero”, which is -273.15 ℃. Obviously, this is not the case, because in our universe, there are simply no microscopic particles that are completely stationary.
On the other hand, according to the “uncertainty principle” in quantum mechanics, the position and momentum of elementary particles in the universe cannot be precisely determined at the same time, and if an object really reaches -273.15°C, it means that elementary particles The position and momentum can be precisely determined at the same time, which violates quantum mechanics, so this temperature is also impossible to reach.
To sum up, -273.15°C is actually the lower limit of temperature defined by scientists according to the actual situation in the universe, and this is the reason why the lowest temperature of the universe is limited to -273.15°C. In theory, any matter in the universe At most, the temperature can only be infinitely close to this temperature, but it is impossible to reach or be lower than this temperature.



GIPHY App Key not set. Please check settings