The researchers say clumps of human cells known as “brain organoids” can grow into millions of new neurons and connect to the nervous system of a new host. When these organs were implanted into the brains of mice, the animals could use their whiskers to receive sensory signals and help generate command signals to guide their behavior.
Dr. Sergiu Pasca, a neuroscientist who led the study, said he and colleagues are now using transplanted human neurons to understand the biological basis of autism, schizophrenia and other developmental disorders . “To address the biology of these diseases, we need more sophisticated models of the human brain,” he added.
In 2009, after medical training in Romania, Dr. Pascal joined Stanford University as a postdoctoral researcher, learning how to create human neurons in a petri dish. He and his colleagues took skin cells from volunteers and put them in special chemicals to prompt them to change traits. These skin cells become more embryonic and can develop into any tissue in the body.
As more chemicals were added, the researchers tricked the cells into developing into neurons. They then observed that when neurons were placed in a petri dish, voltage pulses were fired along the length of the neuron. Dr. Pascal and others performed the same experiment again, this time using skin cells from patients with Timothy syndrome. This is a rare form of autism caused by a single mutation. Not only can this mutation cause serious heart problems, it can also impair language and social skills.
Growing Timothy Syndrome neurons in a petri dish, Dr. Pascal could see many of the differences between them and typical neurons. For example, they produce additional amounts of signaling chemicals like dopamine. But examination of individual cells can only reveal limited clues about the disease. Dr. Pascal suspects that he can learn more by studying thousands of neurons connected together, known as brain organoids.
A new chemical formula allows Dr. Pascal to simulate what’s going on inside the developing brain. When exposed to this medium, skin cells develop into progenitor cells that eventually develop into tangles of neurons in the outer layer of the brain called the cortex. In a later study, Dr. Pascal and his colleagues connected three brain organoids, which consist of cortex, spinal cord, and muscle cells. Studies have shown that stimulating the cortical organ causes muscle cells to contract.
But brain organoids are by no means miniature brains. For one, their neurons remained stunted. Second, they are not as active as neurons in a living brain. “It’s clear that these models still have a lot of limitations,” Dr Pascal said.
With this in mind, scientists have begun implanting organoids into living brains, theorizing that petri dishes limit organoid development. In 2018, neuroscientist Fred Gage and colleagues at the Salk Institute for Biological Studies transplanted brain organoids into the brains of adult mice. As the mouse brains fed human neurons, those neurons continued to develop.
Since then, Dr. Gage and other researchers have implanted organoids into the back of the mice’s brains, an area used to sense signals from the eyes. A study published online in June, which has not yet been peer-reviewed, showed that when mice saw flashes of pulsing white light, neurons in human brain organoids responded in much the same way as the mice’s own cells.
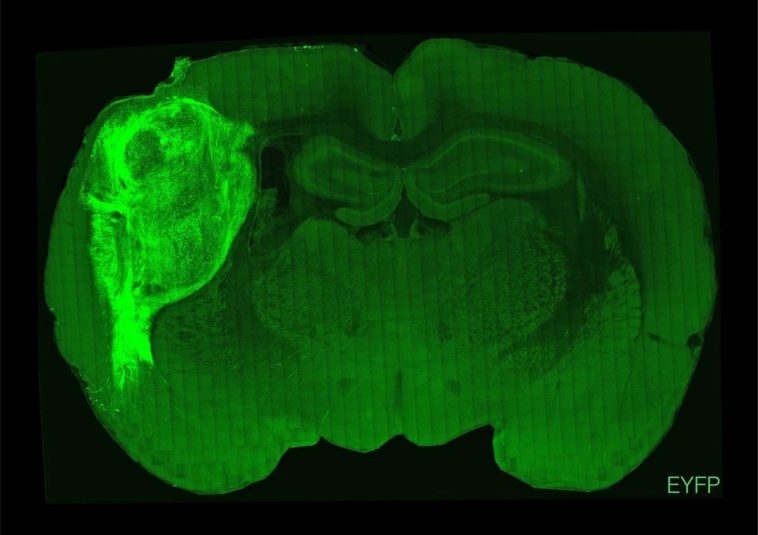
Dr. Pascal and his team are also working on organoid transplants, but they chose to transplant them into young mice. A day or two after the young mice were born, the scientists injected the organoids into an area of the mice’s brain called the somatosensory cortex, which processes touch, pain and other signals from throughout the body. In mice, this area is particularly sensitive to signals from whiskers.
Human neurons continued to develop in the mouse brain until they numbered 3 million, about a third of the cortex on one side of the mouse brain. The results showed that each cell in the organoid grew six times as long as in a petri dish, and the cells also became as active as neurons in the human brain.
More interestingly, these organoids spontaneously wired into the mouse brain. They connect not only with nearby neurons, but also with distant neurons. These connections make human neurons very sensitive to mouse senses. When the researchers blew into the whiskers of mice, the human neurons in the mice responded violently.
Dr. Pascal and others also conducted experiments using indoor drinking fountains to understand how brain organoids affect behavior in rats. After 15 days of training, the mice learned a new skill: They could drink water from a fountain when their brain organoids were stimulated. These human brain organoids are apparently sending messages to areas of the mouse brain that look for reward.
Of course, these species-mixing experiments also raise many ethical questions. Before the study began, Dr. Pascal consulted with experts at Stanford’s Center for Law and Biological Sciences, who wanted him to pay particular attention to the suffering and well-being of animals. “It’s not just how many mice you have in a cage or how well they’re eating,” said Henry Greely, a law professor at Stanford University. “It’s something new, and you don’t even know it’s possible. What will you see.”
Dr. Pascal’s team found no evidence that the mice experienced pain, were more prone to seizures, lost memory, or lost control of their movements. “It turns out that these mice are very well tolerated by human brain organoids,” he said.
Although not involved in the new study, USC neurobiologist Giorgia Quadrato noted that the implantation of human brain organoids did not make mice more human-like. For example, they did not score higher than other mice on learning tests. Cuadrato added: “They are mice, and they are still mice after the organ is implanted. From an ethical point of view, that should be reassuring.”
But if scientists implant human brain organoids into other closely related species, such as monkeys or chimpanzees, this could have serious consequences. “This will be a great opportunity to help develop guidelines to operate within the right ethical framework in the future,” Dr Cuadrato said.
Dr Pascal said the similarities between primates and humans may allow brain organoids to develop more maturely and have a greater impact on the animals’ behavior. But he said: “We do not conduct research like this, nor do we encourage others to do so.”
Instead, he is using brain organoids implanted in mice to study neurological diseases. In one experiment, Dr. Pascal’s team implanted a brain organoid from a patient with Timothy syndrome on one side of a rat’s brain and another brain organoid without the mutation on the other side.
Both organoids continued to grow in the mice. However, neurons in organoids from patients with Timothy syndrome developed twice as many branches, or dendrites, to receive incoming signals. What’s more, these dendrites are shorter.
Dr. Pascal hopes that when mice are implanted with organoids from people with autism and other neurological disorders, he will be able to observe differences in how they behave. Such experiments may help reveal how certain mutations alter the way the brain works.
Dr. Isaac Chen, a neurosurgeon and organoid transplant researcher at the University of Pennsylvania who was not involved in the study, sees another possibility in the new study: helping repair damage to the human brain.
Dr. Chen envisions growing brain organoids from skin cells from patients with damaged cortex and then injecting them into the patient’s brain, where the organoids may eventually continue to grow and connect with healthy neurons. “The idea must already be there,” he said. “The question is, how do we make it happen?”




GIPHY App Key not set. Please check settings